Health Secretary Robert F. Kennedy Jr. announced new limits on who is eligible for COVID #vaccines on Wednesday. This is all happening amid internal turmoil at the Centers for Disease Control and Prevention, the national public health agency that has seen a wave of high-level departures.
Pharmacists no longer have the power to dispense vaccines. Patients who want the #COVID vaccine will need to consult their doctor first rather than book through the pharmacy.
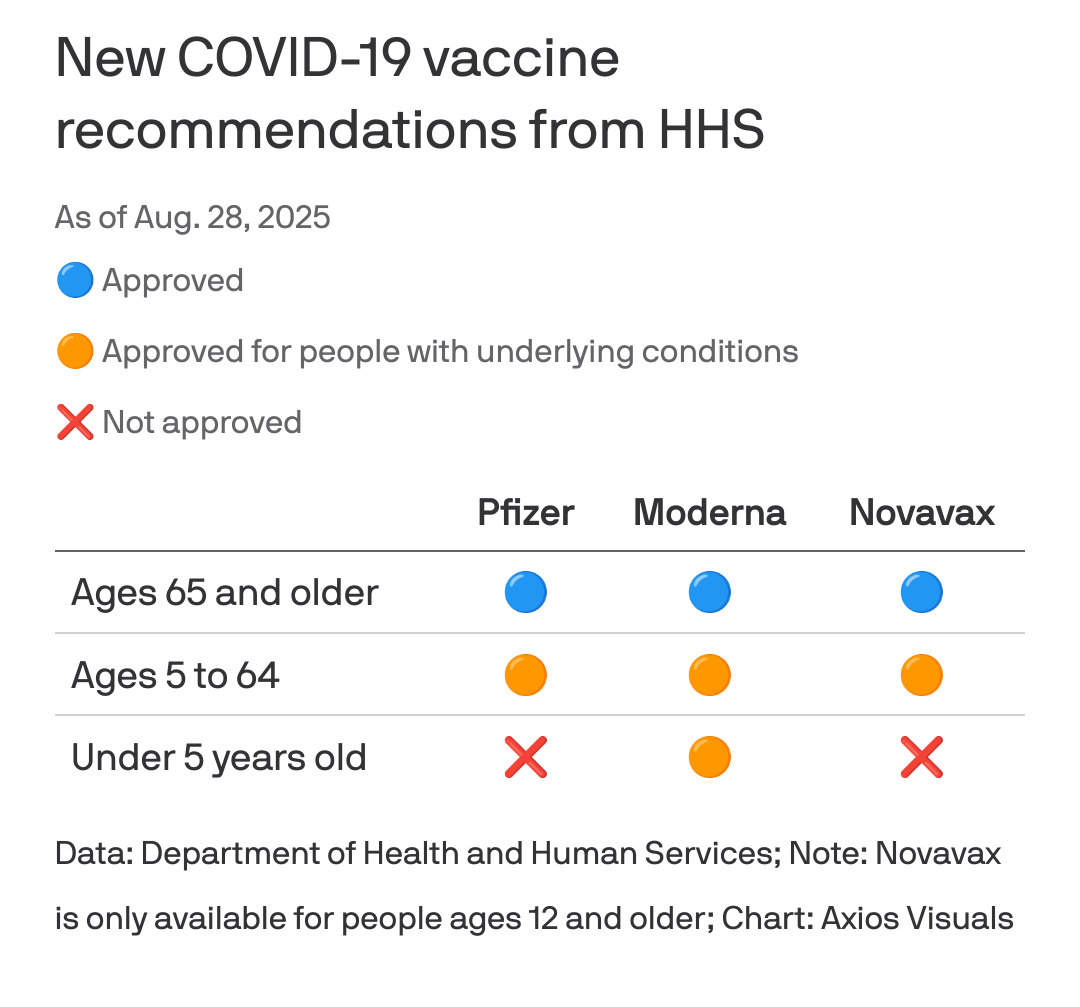
Otherwise, not much changes for individuals 65 years old and up.
Anyone from 5 to 64 years old is eligible for #Pfizer or #Moderna if they have at least one underlying health condition that puts them at risk; the same goes for #Novavax, but only for those 12 years and up
Parents can seek prescriptions for the Moderna vaccine for children 6 months and up with at least one underlying condition.
Axios / https://archive.is/JwtOD#COVID19